


Electronic band structure in one-dimension: A
primer
Everything begins with directional wavefunction overlap
in the context of tight-binding (Hückel theory) invoking the Born-Oppenheimer approximation.
Schrödinger Equation:
Of course y is the wavefunction, m is the electron mass and U is the potential
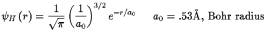
The calculation is exact and analytic if we use a H2+ion.


+

+
Bonding orbitals occur when symmetric solution is used yH(r) + yH(r + R)
Anti-bonding orbitals occur when asymmetric solution is used yH(r) - yH(r + R)


Ebonding
< Eantibonding
