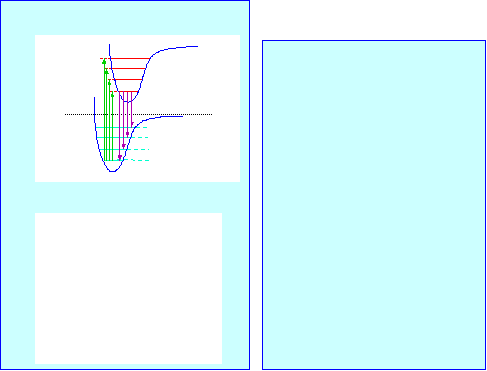
A simple picture of photophysics
in isolated molecules
in isolated molecules
E
f
0
1
2
3
0
1
2
3
E
0-0
E
0-0
=
p
0-3
Emission
p*
0-1
0-2
0-0
0-0
0-1
0-2
0-3
Absorption
(gas phase)

0-3
Photon Energy
0-0
0-1
0-2
0-3
0-0
0-1
0-2
Emission
Absorption
Stokes
shift
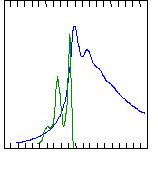
Dilute solutions
in solvent or the solid-state:
Absorption occurs at all sites
but emission dominated by
longest conjugated
segments.
inhomogeneous
broadening
Conjugated
polymers display
Energy (eV)
3.5
2.5
1.5
Absorbance
PPV
Photoluminescence
15 K
T=77 K
(from R.H.
Friend et al.)